What is possible and not possible during the Nativity Fast?
In 2018, the Nativity Fast will begin on November 28. During this period, Orthodox believers prepare to celebrate Christmas...
Chemical properties of iron Let's look at the example of its interaction with typical non-metals - sulfur and oxygen.
Mix iron and sulfur crushed to powder in a Petri dish. Let's heat a steel knitting needle in a flame and touch it to the mixture of reagents. A violent reaction between iron and sulfur is accompanied by the release of heat and light energy. The solid product of the interaction of these substances, iron (II) sulfide, is black. Unlike iron, it is not attracted by a magnet.
Iron reacts with sulfur to form iron(II) sulfide. Let's create the reaction equation:
The reaction of iron with oxygen also requires preheating. Pour quartz sand into a thick-walled vessel. Let's heat a bunch of very thin iron wire - the so-called iron wool - in the flame of a burner. Place the hot wire into a vessel containing oxygen. Iron burns with a dazzling flame, scattering sparks - hot particles of iron scale Fe 3 O 4.
The same reaction also occurs in air, when steel becomes very hot from friction during machining.
When iron burns in oxygen or in air, iron scale is formed:
3Fe + 2O 2 = Fe 3 O 4, Material from the site
or 3Fe + 2O 2 = FeO. Fe 2 O 3 .
Iron oxide is a compound in which iron has different meanings valence.
The passage of both reactions of the connection is accompanied by the release of thermal and light energy.
On this page there is material on the following topics:
Questions about this material:
Lesson objectives:
Equipment: iron (powder, pin, plate), sulfur, oxygen flask, hydrochloric acid, iron(II) sulfate, iron(III) chloride, sodium hydroxide, red and yellow blood salts.
DURING THE CLASSES
I. Organizational moment
II. Checking homework
III. Learning new material
1. Teacher's introduction.
– The importance of iron in life, its role in the history of civilization. One of the most common metals in earth's crust is iron. It began to be used much later than other metals (copper, gold, zinc, lead, tin), which is most likely due to the low similarity of iron ore with the metal. It was very difficult for primitive people to realize that metal could be obtained from ore, which could be successfully used in the manufacture of various objects; this was due to the lack of tools and necessary devices for organizing such a process. Quite a long time passed before man learned to extract iron from ore and make steel and cast iron from it.
At the moment, iron ores are a necessary raw material for ferrous metallurgy, those minerals that no developed industrial country can do without. Annual world production of iron ore is approximately 350,000,000 tons. They are used for smelting iron (carbon content 0.2-0.4%), cast iron (2.5-4% carbon), steel (2.5-1.5% carbon). Steel has the most widespread use in industry than iron and cast iron, which is why there is greater demand for its smelting.
To smelt cast iron from iron ores, blast furnaces are used that run on coal or coke; steel and iron are melted from cast iron in reverberatory open-hearth furnaces, Bessemer converters, or the Thomas method.
Ferrous metals and their alloys are of great importance in the life and development of human society. All kinds of household and consumer items are made of iron. For the construction of ships, aircraft, railways, cars, bridges, railways, various buildings, equipment and other things, hundreds of millions of tons of steel and cast iron are used. There is no branch of agriculture and industry in which iron and its various alloys are not used.
The few minerals commonly found in nature that contain iron are iron ore. Such minerals include: brown iron ore, hematite, magnetite, and others that form large deposits and occupy vast areas.
The chemical relation of magnetite or magnetic iron ore, which has an iron-black color and a unique property - magnetism, is a compound consisting of iron oxide and iron oxide. IN natural environment it can be found both in the form of granular or solid masses, and in the form of well-formed crystals. Iron ore is richest in the metallic iron content of magnetite (up to 72%).
The largest deposits of magnetite ores in our country are located in the Urals, in the Vysokaya, Blagodat, Magnitnaya mountains, in some areas of Siberia - the Angara River basin, Mountain Shoria, on the territory of the Kola Peninsula.
2. Work with the class. Characteristics of iron as a chemical element
a) Position in periodic table:
Exercise 1. Determine the position of iron in the Periodic Table?
Answer: Iron is located in the 4th major period, even row, 8th group, minor group.
b) structure of the atom:
Task 2. Draw the composition and structure of the iron atom, electronic formula and cells.
Answer: Fe +3 2) 8) 14) 2)metal
p = 26
e = 26
n = (56 – 26) = 301s 2 2s 2 2p 6 3s 2 3p 6 3d 6 4s 2
Question. On which layers of iron are the valence electrons located? Why?
Answer. Valence electrons are located on the last and penultimate layers, since this is an element of the secondary subgroup.
Iron is classified as a d-element; it is part of the triad of elements - metals (Fe-Co-Ni);
c) redox properties of iron:
Question. What is iron - an oxidizing agent or a reducing agent? What oxidation states and valence does it exhibit?
Answer:
Fe 0 – 2e = Fe +3) reducing agent
Fe 0 – 3e = Fe +3
s.o.+ 2,+ 3; valency = II and III, valency 7 – does not show;
d) iron compounds:
FeO – basic oxide
Fe(OH) 2 – insoluble base
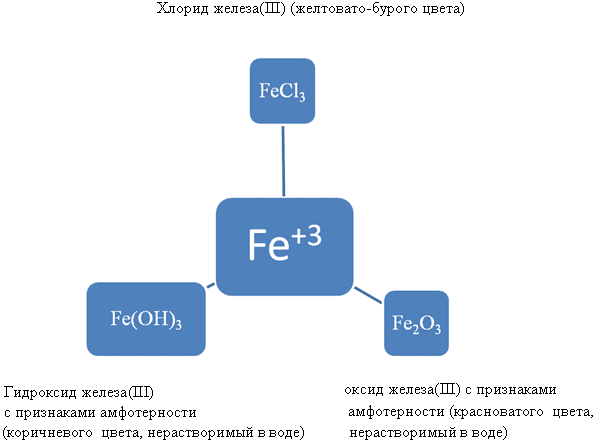
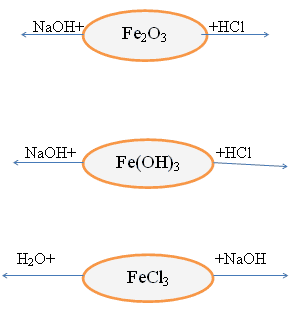
Fe 2 O 3 – oxide with signs of amphotericity
Fe(OH) 3 – a base with signs of amphotericity
Volatile hydrogen compounds are not.
d) being in nature.
Iron is the second most abundant metal in nature (after aluminum). In its free state, iron is found only in meteorites. The most important natural compounds:
FeO*3HO – brown iron ore,
FeO – red iron ore,
FeO (FeO*FeO) – magnetic iron ore,
FeS – iron pyrite (pyrite)
Iron compounds are found in living organisms.
3. Characteristics of the simple substance iron
a) molecular structure, type of bond, type of crystal lattice; (independent)
b) physical properties of iron
Iron is a silver-gray metal, has great malleability, ductility and strong magnetic properties. The density of iron is 7.87 g/cm 3, the melting point is 1539 t o C.
c) chemical properties of iron:
Iron atoms donate electrons in reactions and exhibit oxidation states of + 2, + 3 and sometimes + 6.
In reactions, iron is a reducing agent. However, at ordinary temperatures it does not interact even with the most active oxidizing agents (halogens, oxygen, sulfur), but when heated it becomes active and reacts with them:
2Fe +3Cl 2 = 2FeCl 3 Iron(III) chloride
3Fe + 2O 2 = Fe 2 O 3 (FeO*Fe O) Iron(III) oxide
Fe +S = FeS Iron(II) sulfide
At very high temperature iron reacts with carbon, silicon and phosphorus.
3Fe + C = Fe 3 C Iron carbide (cementite)
3Fe + Si = Fe 3 Si Iron silicide
3Fe + 2P = Fe 3 P 2 Iron phosphide
Iron reacts with complex substances.
In humid air, iron quickly acidifies (corrodes):
4Fe + 3O 2 + 6H 2 O = 4Fe(OH) 3
Fe(OH) 3 ––> FeOOH + H 2 O
Rust
Iron is in the middle of the electrochemical voltage series of metals, therefore it is a metal average activity. The reducing ability of iron is less than that of alkali, alkaline earth metals and aluminum. Only at high temperatures does hot iron react with water:
3Fe + 4H 2 O = Fe 3 O 4 + 4H 2
Iron reacts with dilute sulfur and hydrochloric acids, displacing hydrogen from them:
Fe + 2HCl = FeCl 2 + H 2
Fe + H 2 SO 4 = FeSO 4 + H 2
Fe 0 + 2H + = Fe 2+ + H 2 0
At ordinary temperatures, iron does not interact with concentrated sulfuric acid, since it is passivated by it. When heated, concentrated sulfuric acid oxidizes iron to iron(III) sulfate:
2Fe + 6H 2 SO 4 = Fe 2 (SO 4) 3 + 3SO 2 + 6H 2 O
Dilute nitric acid oxidizes iron to iron(III) nitrate:
Fe + 4HNO 3 = Fe(NO 3) 3 + NO + 2H 2 O
Concentrated nitric acid passivates iron.
From salt solutions, iron displaces metals that are located to the right of it in the electrochemical voltage series:
Fe + CuSO 4 = FeSO 4 + Cu,
d) use of iron (on your own)
e) receiving (together with students)
In industry, iron is obtained by reducing it from iron ores with carbon (coke) and carbon monoxide (II) in blast furnaces.
The chemistry of the blast furnace process is as follows:
C + O = CO
CO + C = 2CO
3Fe 2 O 3 + CO = 2Fe 3 O 4 + CO 2
Fe 3 O 4 + CO = 3FeO + CO 2
FeO + CO = Fe + CO 2
4. Iron compounds
Chemical properties of these compounds.

Addition. Iron(II) compounds are unstable, they can oxidize and turn into iron(III) compounds
Fe +2 Cl 2 + Cl 2 = Fe +3 Cl 3 make up redox house
Fe +2 (OH) + H 2 O + O 2 = Fe +3 (OH) 3 schemes, equalize.
Chemical properties of these compounds
Also, a qualitative reaction to Fe +2 is the reaction of iron(II) salts with a substance called red blood salt K3 - this is a complex compound.
3FeCl + 2K 3 = Fe 3,"de":["HY7CqWc6WfE"],"es":["zhomCMDQurk","cFsKQvX-sH8","o2GN1FUPJH8"],"pt":["uIEIrciTwOc","0AyoZ_UU2yY" ],"fr":["31nvESQbbG8","1FmdeA4K5gg"],"ro":["6HNFpOmTS2c","YAlG-FBbUWc","YAlG-FBbUWc"])